Imperfections in Solids
A crystal in which the constituent particles have a definite repeating arrangement is called an ideal crystal. However, when some constituent particles do not occupy theoretical positions, the crystal structure shows some deviations called defects.
In short- Imperfection or defect in a crystal is the departure of the constituent particles of the crystal from its regular position in the lattice.
Type of defects in solids
Point defects
Point defects arise due to irregularities or deviations from ideal arrangement around a point or an atom.
Line defects
Line defects are the deviations from ideal arrangement in entire rows of lattice points. However, we shall confine our discussion to point defects only.
Types of Point Defects
- Stoichiometric defects
- Non-stoichiometric defects
- Impurity defects
Stoichiometric Defects
Stoichiometric compounds are those compounds in which the number of positive and negative ions are in the same ratio as indicated by their chemical formula.
These defects are also called Intrinsic or Thermodynamic defects because the number of defects depend upon the temperature. At absolute zero, the ions are arranged in a regular fashion in the crystal. However, on increasing temperature, the chance for an ion to escape from the lattice site and thus to create a vacant site or a defect increase.
Stoichiometric defects are mainly of two types-
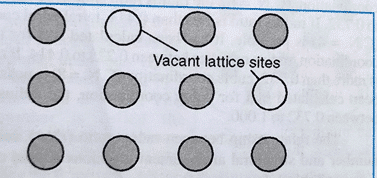
Vacancy Defect
This type of defect arises when some constituent particles are missing from their regular lattice positions. The unoccupied positions are called Vacancies. Due to this type of defect, the density of the substance decreases. This defect can also develop on heating a substance.
Interstitial Defect
This type of defect arises when some constituent particles (atoms or molecules) occupy an Interstitial site. Density of the substance increases due to Interstitial defect.
The two types of defects described above can be shown by non-ionic solids. In case of ionic solids, electrical neutrality is also to be maintained.

Therefore, rather than simple vacancy or interstitial defects, they show these defects as Schottky and Frenkel defects.
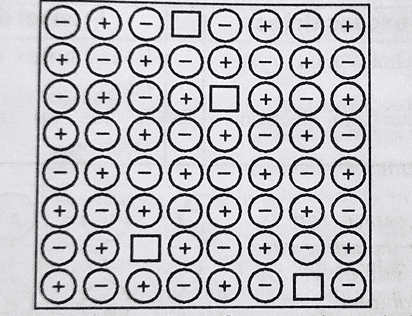
Schottky Defects
This defect discovered by a German Scientist Schottky in 1930 is basically a vacancy defect. Schottky defects in ionic solids arise in a crystal when the same number of cations and anions are missing from their normal lattice sites and a result, pairs of holes are formed.
Conditions causing Schottky defects
This type of defect is generally observed in case of strongly ionic compounds:
- With high coordination number
- Having positive and negative ions of same size
Examples of compounds having this type of defect are:
NaCl, KCl, KBr, CsCl and AgBr.
Number of ions having one Schottky defect in NaCl ionic crystals
It has been observed that there are about 106 Schottky pairs per cm3 at room temperature (one cm3 has about 1022 ions) or about one Schottky defect per 1016 ions in NaCl.
Frenkel Defects
This defect was discovered by a Russian scientist Frenkel in 1926. This is a hybrid type of defect arising from the combination of Schottky defect and interstitial defect.
This type of defect arises due to the presence of a hole in the cationic lattice site and the cation occupying an interstitial position.
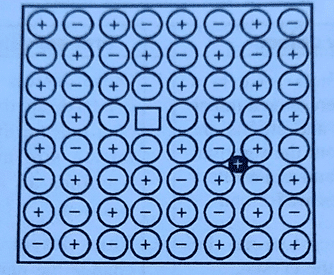
For example, in the crystal lattice of ZnS and AgBr, the Zn2+ and Ag+ ions occupy interstitial sites and leave behind a hole. Frenkel defects creates a vacancy defect at its original site and an interstitial defect at its new location. Frenkel defect is also called Dislocation defect.
Condition causing Frenkel defects
This defect generally occurs in compounds:
- Having low coordination number
- In which anions are much larger than cations
It may be noted that- AgBr shows Frenkel as well as Schottky defect.
You May Also Like-
What Do you know about Solid State?
What do you know about Solid Waste??
Processing of solid waste and On-site Handling by engineering System
What do you know about Hazardous Waste??
Landfill Disposal of Solid Waste
Right Understanding
Right Understanding We all know that the Human Desire is to be in continuous happiness which is the need of I (self). But do you know, from where Continuous happiness will come? No, right! So continuous happiness is to be in Right Understanding, Right Feeling, and Right Thought that is Activity of I (Self). Do…
Where We Are
Where We Are (Self-Evolution) We exist as human being. We want to live a fulfilling life. We have some desires and we have some programs for the fulfilment of it. We need to understand our basic aspiration and program for its fulfillment correctly and comprehensively. Only then, we can ensure fulfillment. We should explore ourselves…
Highway Construction
Highway Construction Embankment Construction Materials and General Requirements The materials used in embankments, subgrades, earthen, shoulders, and miscellaneous backfills shall be soil, moorum, gravel, a mixture of these. Clay having liquid limit exceeding 70 and plasticity index exceeding 45; shall be considered unsuitable for embankment. Sub-grade and top 500mm portion of the embankment just below…
Special Concretes
Special Concretes Concrete is most vital material in modern construction. In addition to normal concrete, other varieties in use are, high strength and high-performance concrete, self-compacting, lightweight, high density, fiber reinforced, polymer, colored concrete, etc. The making of concrete is an art as well as a science. Special types of concrete are those with out-of-the-ordinary…
Marketing Practices
Marketing Practices Success in the world of business, no matter how you earn it, you have to rule on the marketplace. Although luck plays a role in the outcome of the market strategies. In the business decisions, there should be the understanding of market otherwise the failure will take place by the marked decisions. While…
Risk Analysis
Risk Analysis The risk that remains after the implementation of controls is called the residual risk. All systems will have residual risk because it is virtually impossible to completely eliminate risk to an IT system. In other words, we can say that there are two main parts of the security risk analysis known as Quantitative…










